The reactants are the one to the left side, so they are Cu and HNO₃.
Their coefficients are 3 and 8, so for each 3 Cu that reacts, we will need 8 HNO₃
The Limiting reactant is the one that we have less considering the proportions they will react.
Let's see the two possible cases.
If we have 1 mol of Cu, we need to divide it by its coefficient and multiply it by the coefficient of HNO₃:
HNO₃ --- Cu
8 --- 3
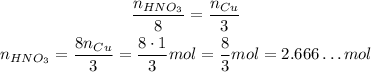
This means that we need about 2.7 mol of HNO₃ to react all the 1 mol of Cu we have.
Since we have 1 mol of HNO₃, we don't have enough.
If we make the other way around, we will get the following:
1 mol of HNO₃, so we need:
Cu --- HNO₃
3 --- 8
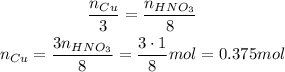
So, we would need 0.375 mol of Cu to react with all 1 mol of HNO₃ we have, and since we have 1 mol of Cu, this is enough.
So, we have two pos