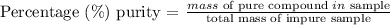
Now purity is the effective percentage of pure reactant in the total mass.
The rest is inert impurities.
They ask you to calculate the purity of the carbonate, so you have to focus on CaCO3
Let's calculate with the reaction:
CaCO3 (s) -----------------> CaO (s) + CO2 (g)
x 88.0 g
100 g CaCO3 ------- 44 g CO2
x ------- 88 g CO2
x = 200.16 g
Sample has 250 g of impurities. So:
Purity % = (200.16 g / 250 g impure) x 100 = 80.0 % of purity