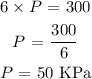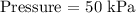
Here, we want to use the proportionality to get the value of the pressure of the gas
From the question, we have it that the pressure and the volume are inversely related at constant temperature
Let us have the pressure as P, volume as V and temperature as T
Thus, we have the relationship as follows;
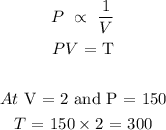
When V = 6, we have P as follows;