1) List known values
Ketamine solution: 10.0% (w/v) solution
Mass of Ketamine: 30mg
2) List unknown values
The volume of solution.
3) Set the equation
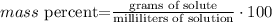
4) Convert mg to g.
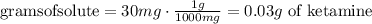
5) Plug in known quantities and solve for volume.
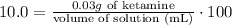
Rearrange algebraically
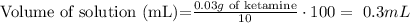
The volume that contains 30 mg of ketamine is 0.3mL
.