We have the density of chloroform which is 1.48 g/cm^3 and the volume required which is 35.0 mL.
To find the mass, we apply the following formula:
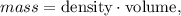
But you should know that cubic centimeters (cm^3) are the same that milliliters (mL).
Doing the calculations, we obtain that:
![\text{mass}=1.48\frac{g}{\operatorname{cm}^3}\cdot35.0cm^3=51.80\text{ g.}]()
The mass of chloroform is 51.8 grams.