ANSWER
Step-by-step explanation
Given that;
The number of moles of NH3 is 3 moles
The number of moles of H2 is 1 mole
The number of moles of N2 is 2 moles
At equilibrium, the concentration of ammonia is 1.4 moles/L
To find the value of Kc, follow the steps below
Step 1: Write the balanced equation of the reaction
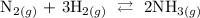
Step 2: Write the equation of the reaction in terms of Kc
![\text{ K}_C\text{ = }\frac{[\text{ NH}_3]^2}{[N_2]\text{ \lbrack H}_2]^3}](https://img.qammunity.org/2023/formulas/chemistry/college/9rzesxec346vs5wbwfu3lwxp1th93ogltj.png)
Step 3: Find the concentration of each reactant at equilibrium using a stoichiometry ratio
From the reaction above, you will see that 1 mole of Nitrogen reacts with 3 moles of hydrogen to give 2 moles of ammonia.
Let x represents the concentration of nitrogen at equilibrium
Recall, that the concentration of ammonia at equilibrium is 1.4 moles/L
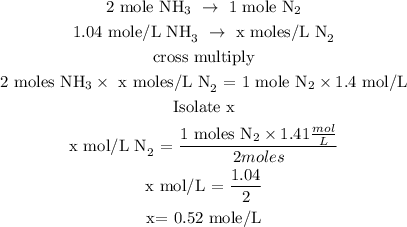
Since the initial number of moles of nitrogen is 1 mole, hence, the concentration of nitrogen at equilibrium is calculated below as
Concentration at equilibrium = 1 -0.52
Concentration of nitrogen at equilibrium = 0.48 mole/L
The next step is to find the concentration of hydrogen at equilibrium
Let y represent the mole of hydrogen at equilibrium
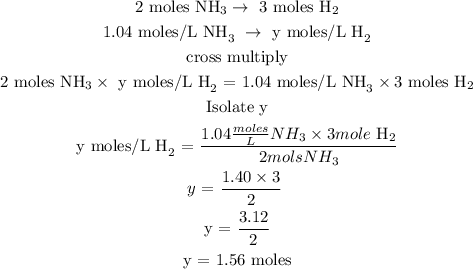
Since the initial concentration of hydrogen is 2 moles, hence the concentration of hydrogen at equilibrium can be calculated below as
Concentration at equilibrium = 2 - 1.56
Concentration at equilibrium = 0.44 mole/L
Step 4: Find the value of Kc using the equation in step 2
![\text{ Kc = }\frac{[NH_3]^2}{[N_2]\text{ \lbrack H}_2]^3}](https://img.qammunity.org/2023/formulas/chemistry/college/gwzvzvwdestqfmkuu1w5w434zmhwgdrn0w.png)
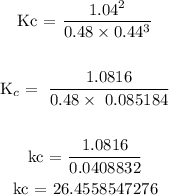
Hence, the value of Kc is 26