we have that
In this problem, we have an equation of the form
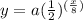
where
y ------> milligrams of Iodine-131 left in the body
x -----> number of days
a ----> initial value
a=10 mg
substitute
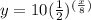
Part 2
For x=4 days
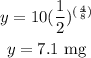
Part 3
y < 0.5 mg
we have the inequality
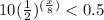
solve the inequality
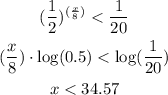
therefore
the number of days must be less than 35 days