Answer:
1.04 L.
Step-by-step explanation:
What is given?
Temperature 1 (T1) = 240 K.
Pressure 1 (P1) = 670 mmHg.
Volume 1 (V1) = 1.28 L.
Temperature 2 (T2) = 198 K.
Pressure 2 (P2) = 680 mmHg.
What do we need? Volume 2 (V2).
Step-by-step solution:
To solve this problem, we have to use the combined gas law. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas. For a combined gas law problem, only the amount of gas is held constant. The formula of combined gas law is:
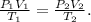
We need to find the volume 2 (V2), so let's solve for this unknown value and let's replace the given data that we have:
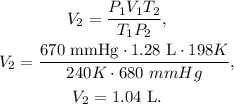
The final volume for this case would be 1.04 L, the volume is being reduced.