So,
The Charles law states that, at a constant pressure, the initial and final volume and temperature of a gas is described with the following equation:
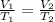
In this question, we're asked to find the value of V2.
Replacing our values:
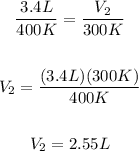
Therefore, the final volume is 2.55L.