The heat abvsorved by a substance is calculated according to the following equation:
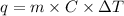
Where q is the heat, m is the mass of the substance, C is the specific heat and AT is the temperature increment. Therefore we have all the required data to solve this problem:
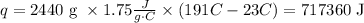
Just be sure all quantities are in the corresponding units