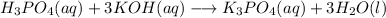
When we balance a reaction (of neutralization in this case) like this, first, focus on the main element that forms hydroxides and acids. In this reaction, those elements are P and K.
We must have the same number of atoms on the right and on the left.
We have 1 P on both sides. Now the K aren't equal, so we multiply by 3 on the left side of the reaction.
After that, we focus on water to balance the Hydrogen and Oxygen elements. On the left, we have 6 H and 7 O, so we multiply H2O by 3 on the right.
To complete the question, you must fill in the gaps.
Answer: a. 1,3,1,3