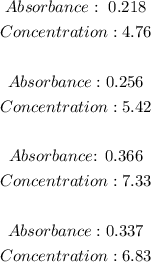
Answer:
To calculate the concentration [FeNCS+2] we use the data shown in the curve (Absorbance vs Concentration).
As we can see, the linear equation for absorbance at 447.1nm, given the values of m and b is:
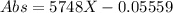
Where X is the concentration [FeNCS+2] in mol/L.
In the table we are given the values of absorbance and we need to calculate the corresponding concentration. Therefore we rearrange the equation:
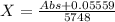
Now we only have to use the absorbance values given in the table to calculate the concentration. The values and results are: