Answer: 59.41 grams of Na2S
Explanation
Na2S will be formed from the chemical reaction below :

We are given :
• Mass of Na = 35.0 g rams
,
• Molar mass Na = 22,989769 g/mol
(1) Calculate moles Na
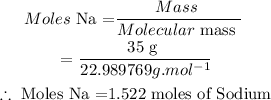
(2) From stoichiometry, the balanced equation informs us that :
2 mole Na will produce 1 mole Na2S
Then , 1.522 moles Na will produce X moles Na2S
Therefore, X moles Na2S = (1.522mole Na * 1 mole Na2S)/ 2 moles Na
=0.761 moles on Na2S will be produced .
(3) Calculate Mass of Na2S
• Molecular mass Na2S = 78,0452 g/mol
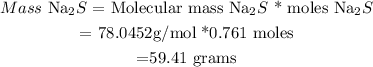
This means that 59.41 grams of Na2S will be produced from 35 g Na.