In order to calculate the mass, we can use the formula below:
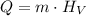
Where Q is the heat energy, m is the mass and HV is the latent heatof pvaporizaion.
Since the latent heat is given in J/g, themas s will be used in g and the energy in J.
So we have:
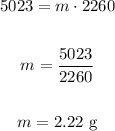
Therefore the mass is 2.22 grams.