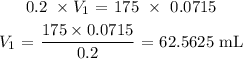Answer:
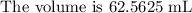
Explanation;
Here, we want to get the volume of KBr that would be required
Mathematically, from the dilution formula, we have it that:
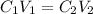
We have it that:
C1 is the concentration of the KBr initially before dilution with water which is 0.2M
V1 is the initial volume that we want to calculate
C2 is the final concentration after dilution which is 0.0715 M
V2 is the final volume which is 175mL
Substituting the values, we have it that: