Answer:
2.295 moles of O2 are needed.
Step-by-step explanation:
1st) It is necessary to write and balance the chemical reaction:
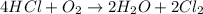
2nd) From the balanced reaction we can see that to produce 2 moles of chlorine gas (Cl2), 1 mole of oxygen gas (O2) is needed. So, with a mathematical rule of three we can calculate the amount of oxygen gas moles needed to produce 4.59 moles of Cl2:
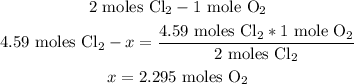
Finally, 2.295 moles of O2 are needed