Answer:
120grams. Option B is correct
Explanations
The formula for calculating the molarity of a solution is expressed as:
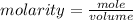
Given the following parameters
• Volume of solution = 1.5L
,
• Molarity = 0.50M
Determine the moles of calcium nitrate
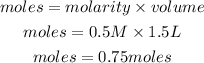
Determine the mass of calcium nitrate
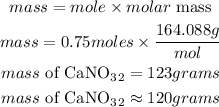
Hence the amount of calcium nitrate needed in correct significant figures is 120grams