First, we have to calculate the number of moles of 1.50 grams of glucose, using its molar mass which is 180 g/mol (you can calculate it using the periodic table and doing the algebraic sum):
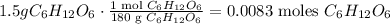
And now, let's use Avogadro's number, which is 6.022 x 10^(23) /mol. This number is telling us that there are 6.022 x 10^(23) atoms or molecules in 1 mol. The number of atoms of glucose is:
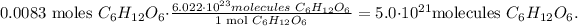
To find the number of atoms in C, you can see that there are 6 atoms of this in glucose, so we're going to calculate:
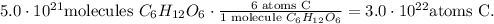
For hydrogen, we have 12 atoms of this in glucose:
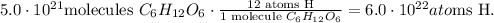
And finally, we have 6 atoms of oxygen in glucose:
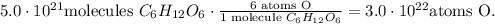
The answer is that we have 3.0 x 10^(22) atoms of oxygen (O), 3.0 x 10^(22) atoms of carbon (C), and 6 x 10^(22) atoms of hydrogen (H).