Answer:
We have the following chemical reaction:
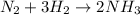
First we calculate the moles of hydrogen that react. For this we use its molar mass:
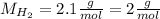
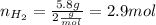
Now, we know that for every 3 moles of H2 we need 1 mol of N2, so we calculate the moles of N2:
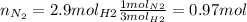
And now we use the nitrogen's molar mass to calculate the grams:
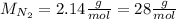
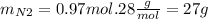
So the answer is 27g