The question requires us to calculate the amount of atoms in 970 kg of copper (Cu).
To solve this question, we need to remember the Avogadro's number, which defines that the number of units (particles, atoms, molecules etc.) in one mole of any substance is equal to 6.02 x 10^23. We can write this information as it follows:
1 mol Cu --------------- 6.02 x 10^23 atoms of Cu
Thus, if we know the mass contained in one mol of Cu, we can calculate the amount of atoms in any given mass of this substance.
The atomic mass of copper is 63.546 u, then we can consider the amount of copper in one mol as 63.546 g.
Now, we write down the information we have (Avogadro's number, molar mass of Cu, 63.546 g, and given mass of Cu, 970 kg) and calculate the required value (amount of atoms in the given mass of Cu):
1 mol Cu ---------- 63.546 g Cu --------------- 6.02 x 10^23 atoms of Cu
970 x 10^3 g Cu ---------- x
(note that we need to convert the given mass of Cu from kilograms into grams)
Now, solving for x, we have:
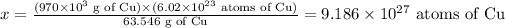
Therefore, there are 9.186 x 10^27 atoms of copper in 970 kg of this element.