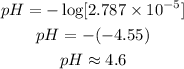Explanations:
a) The formula for calculating the molarity of a solution is expressed according to the formula shown below:
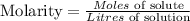
Given the following parameters;
Moles of the solution = 0.0000209 mol
Volume of solution = 750mL
Convert the volume to litres
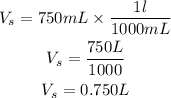
Get the required molarity using the formula above;
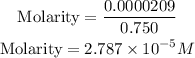
b) To get the pH of the acid in the drink, we will use the formula;
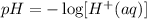
The expression in the square bracket is the molarity of the solution. Substituting the given molarity into the formula: