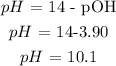Answer:
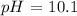
Step-by-step explanation:
We start off by writing the ionization equation
We have this as follows:
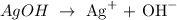
Let us have the concentration of the silver and hydroxide ions as x M
Thus, we have this as:
![\begin{gathered} K_(sp)\text{ = \lbrack Ag}^+]\text{ + \lbrack OH}^-] \\ 1.55\text{ }*\text{ 10}^(-8)\text{ = x}* x \\ x^2\text{ = 1.55 }*\text{ 10}^(-8) \\ x\text{ = }\sqrt{(1.55\text{ }*10^(-8))} \\ x\text{ = 0.0001245} \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/rrdvj83pqzxzrsblx0aw8eh7gf95ftw0t5.png)
Thus, we have the value of x as 0.0001245
Mathematically:
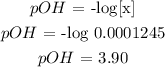
However: