Answer:
There are 1.01x10^24 molecules of I2.
Step-by-step explanation:
1st) It is necessary to use the molar mass of I2 to convert the 425g to moles:
- I2 molar mass: 254g/mol
- Conversion from grams to moles:
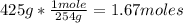
Now we know that there are 1.67 moles of I2 in 425g.
2nd) To calculate the molecules, it is necessary to use the Avogadro's number. The Avogadro's number is equal to 6.022*10^23 particles (in this case, molecules) and it is the number of particles in 1 mole of substance.
With the Avogadro's number and the moles of I2, we can calculate the number of molecules, using a mathematical rule of three:
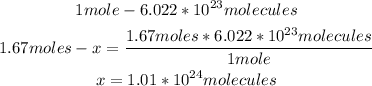
So, there are 1.01x10^24 molecules of I2.