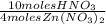Answer:
The mole ratio is incorrect.
Step-by-step explanation:
It is incorrect, because the mole ratio is derived from the stoichiometry of the reaction, in this case, the relation between HNO3 and Zn(NO3)2 is that, from 10 moles of HNO3, 4 moles of Zn(NO3)2 are produced, so the correct mole ratio is: