To determine which element is reduced and which is oxidized, we must see what the oxidation states of each element are before and after the reaction.
The element that increases its oxidation state will be the one that is oxidized and therefore will be the reducing agent. This element loses electrons
The element that decreases its oxidation state will be the one that is reduced and therefore will be the oxidizing agent. This element gains electrons.
Let's see what the oxidation states are:
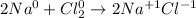
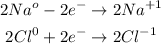
We see that sodium loses electrons and oxidizes. While chlorine gains electrons and is reduced. Therefore, the answer will be the first option:
Answer: Na is a reducing agent because Na loses electrons and is oxidized to Na+