1) Chemical equation (unbalanced).
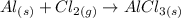
List the elements in the reactants and in the products.
Reactants
Al: 1
Cl: 2
Products
Al: 1
Cl: 3
2) Balance Cl.
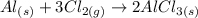
Reactants
Al: 1
Cl: 6
Products
Al: 2
Cl: 6
3) Balance Al.
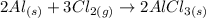
Reactants
Al: 2
Cl: 6
Products
Al: 2
Cl: 6
The correct coefficient for aluminum chloride (AlCl3) is 2.
.