Answer
(e) 6.20
Step-by-step explanation
The pH of the solution can be calculated using the formula below:
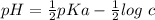
Note that:
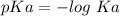
Put Ka = 4.5 x 10⁻¹³ into the pKa formula:
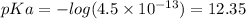
Put pKa = 12.35 and c = 0.90 into the pH formula above:
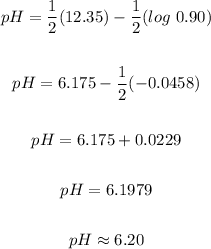
The pH = 6.20
The correct option is (e) 6.20