Step 1 - Understanding the molar volume
Every gas, in certain conditions known as STP conditions, occupies the same volume per mol. This is known as the molar volume, and corresponds to 22.4 L/mol. This means that each mole of a gas occupies 22.4 L (at STP).
Step 2 - Using the molar volume to solve the problem
Since we already know the relation between moles and volume in the STP, we can set the following proportion:
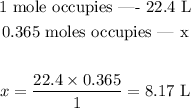
0.365 moles of O2 gas at STP occupies thus 8.17 L.