Answer:
• Reaction:
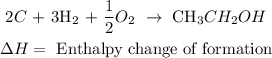
• Reaction:
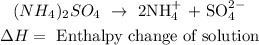
• Reaction:
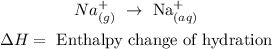
• Reaction:
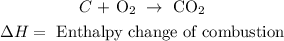
• Reaction:
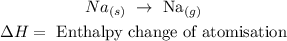
• Reaction:
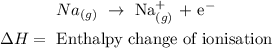
• Reaction:
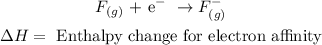
Step-by-step explanation:
• Enthalpy change of ,combustion,: is the enthalpy involved in the process of in which a compound burns in the presence of oxygen (or air).
,
• Enthalpy change of ,formation,: this enthalpy change is the one involved when 2 or more reactants combine to form a product.
,
• Enthalpy change of ,solution,: is the energy present in the dissolution of a substance in a solvent.
,
• Enthalpy change of ,hydration,: it is the energy that intervenes when a substance in gaseous atate becomes part of the aqueous solution.
,
• Enthalpy change of ,atomisation,: this is necessary to form gaseous atoms from the element under standard conditions.
,
• Enthalpy change of ,ionisation,: is the energy involved when an ion is formed.
,
• Enthalpy change for ,electron affinity,: is the energy released when a neutral gaseous atom takes on an electron and forms a negative ion.