Answer
The energy needed = 5790 J
Step-by-step explanation
Given:
Raise in temperature, ΔT = 50 °C
Mass of copper sample, m = 300.0 grams
Specific heat, c of Cu = 0.386 J/g.°C
What to find:
The quantity of energy, Q needed.
Step-by-step solution:
The energy, Q needed can be calculated using the formula;
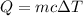
Plugging the values of the parameters into the formula;
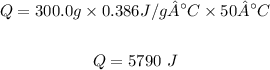
Therefore, the quantity of energy, Q needed is 5790 J