1) Write the chemical equation.
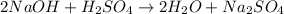
2) Convert the mass of Na2SO4 to moles of Na2SO4.
The molar mass of Na2SO4 is 142.0421 g/mol.
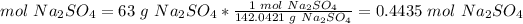
3) Moles of NaOH needed
The molar ratio between NaOH and Na2SO4 is 2 mol NaOH: 1 mol Na2SO4.
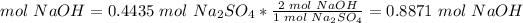
0.8871 mol NaOH is required to form 63 g Na2SO4.