Answer: 483 mL of the cleaning solution are used to clean hospital equipment
Step-by-step explanation:
The question requires us to calculate the volume, in mL, of solution is used to clean hospital equipment, given that 415g of this solution are used and the specific gravity of the solution is 0.860.
Measurements > Density
Specific gravity is defined as the ratio between the density of a given substance to the density of a reference material, such as water:
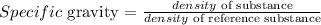
The density of a substance is defined as the ratio between the mass and the volume of this substance:
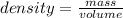
Considering the reference substance as water and its density as 1.00 g/mL, we can determine the density of the substance which specific gravity is 0.860:
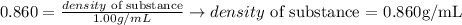
Thus, taking water as the reference substance, we can say that the density of the cleaning solution is 0.860 g/mL.
Now that we know the density of the cleaning solution (0.860 g/mL) and the mass of solution that is used to clean hospital equipment (415g), we can calculate the volume of solution that is used to clean the equipment:

Therefore, 483 mL of the cleaning solution are used to clean hospital equipment.