ANSWER
The number of moles of hydrogen is 6.7 moles
The number of moles of oxygen is 3.3 moes
Step-by-step explanation
Given that;
The total number of moles of gases is 10
The mole fraction of hydrogen is 0.67
Follow the steps below to find the number of moles of each gas
Recall, that the total mole fractions all of the gases in container must be 1
Mathematically
Mole fraction of hydrogen + mole fraction of oxygen = 1
The next step is to find the mole fraction of oxygen
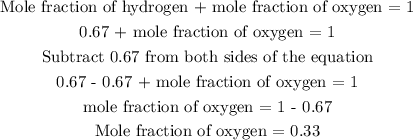
The mole fraction of oxygen is 0.33
The next step os to find the number of moles of each gas in the container using the below formula
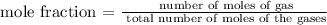
For hydrogen gas

Therefore, the number of moles of hydroges gas in the mixture is 6.7 moles
For oxygen

The number of moles of oxygen is the mixture is 3.3 moles