Step 1 - Understanding molar mass
The molar mass of a substance is the mass, in grams, of one mole of that substance. Let's take water as an example.
The molar mass of water is 18 g/mole. This means that in 18 g of water there is one mole of water, which corresponds to 6.02*10^23 molecules of water.
The molar mass of sucrose is 342.3 g/mole, which means 1 mole of sucrose weights 342.3 g.
Step 2 - Calculating the number of moles of sucrose
We can use the molar mass to set the following proportion:
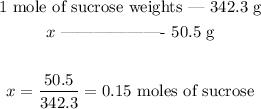
There are thus 0.15 moles of sucrose in 50.5 g of it.