Answer:
The percent composition is 21% N, 6% H, 24% S and 49% O.
Step-by-step explanation:
1st) The molar mass of (NH4)2SO4 is 132g/mol, and it represents the 100% of the mass composition.
In 1 mole of (NH4)2SO4, there are:
- 2 moles of N.
- 8 moles of H.
- 1 mole of S.
- 4 moles of O.
2nd) It is necessary to calculate the mass of each element, multiplying its molar mass by the number of moles:
- 2 moles of N (14g/mol) = 28g
- 8 moles of H (1g/mol) = 8g
- 1 mole of S (32g/mol) = 32g
- 4 moles of O (16g/mol) = 64g
3rd) With a mathematical rule of three we can calculate the percent composition of each element in the molecule of (NH4)2SO4:
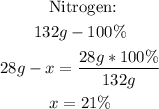
![\begin{gathered} \text{ Hydrogen:} \\ 132g-100\operatorname{\%} \\ 8g-x=\frac{8g*100\operatorname{\%}}{132g} \\ x=6\% \end{gathered}]()
![\begin{gathered} \text{ Sulfur:} \\ 132g-100\operatorname{\%} \\ 32g-x=\frac{32g*100\operatorname{\%}}{132g} \\ x=24\% \end{gathered}]()
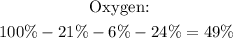
In this case, we can calculate the percent composition of Oxygen by subtracting the other percentages, since the total must be 100%.
So, the percent composition is 21% N, 6% H, 24% S and 49% O.