We are told that starch consists of glucose polymer, so we can assume that the caloric value of starch will be equal to the caloric value of glucose, that is, 3.9kcal/g.
Now to determine the kcal and kJ there were in the potato we must calculate the mass of starch present in that potato. We are told that it is 92% starch, therefore the mass of starch in the potato will be:
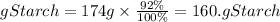
We have that in the potato there are 160.08 grams of starch. By multiplying it by the caloric value we will have the kcal that were in the potato, assuming that the rest of the ingredients do not contribute caloric value, or it is insignificant.
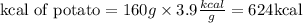
To calculate the kJ we must make the conversion using the relationship that 1 cal is equal to 4.184 joules:
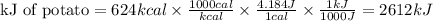
In the potato, there were 624 kcal of energy or 2612kJ of energy.