To determine the pH, we need to find the concentration of hydrogen and hydroxide ions in a solution. It can be calculated with the following formula.
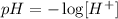
Givens.
• The concentration of Sulfur Dioxide in the atmosphere is 0.12 ppm.
The chemical equation about the situation is
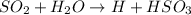
We can the hydrogen ions products whose concentration we need to find.
We know that 1 ppm is equivalent to 1x10^-6 atm. Let's find the pressure of SO2 in atm.
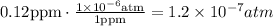
Then, determine the equilibrium concentration Ka.
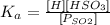
As you can observe, the pressure in the atmosphere is the concentration of SO2. Also, we know that the constant of equilibrium of HSO3 is 1.3x10^-2, so
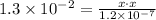
Then, solve for x.
![\begin{gathered} x^2=1.3*10^(-2)\cdot1.2*10^(-7) \\ x=\sqrt[]{1.56*10^(-9)_{}} \\ x=3*10^(-5) \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/3zkzyr6wf4egmo7652rj2p8uax8xduef0a.png)
Where x represents the concentration of hydrogen ions and HSO3. Now, we are able to find the pH.
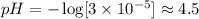
Therefore, the pH of the rainwater due to this pollutant is 4.5.