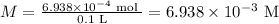Answer
6.938 x 10⁻³ M
Procedure
Data
Mass of sodium iodide (NaI) = 0.104 grams
Volume of the solution = 100 mL = 0.1 L
Molarity of aqueous solution of silver nitrate (AgNO₃) = 47 mM = 0.047M
The molecular mass of sodium iodide is 149.89 g/mol.
Step 1
Find the chemical reaction:
NaI + AgNO₃ -> NaNO₃ + AgI
Step 2
Calculate the number of moles of sodium iodide
Moles NaI = mass NaI / Molar mass NaI
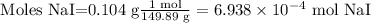
Step 3
For 1 mole AgNO₃ consumed, we need 1 mole NaI to produce 1 mole of AgI and 1 mole NaNO₃
The sodium iodide will dissociate as followed:
NaI(aq) → Na+(aq) + I-(aq)
Step 4
Calculate iodide ions
For 1 mole NaI, we have 1 mole of Na⁺. Therefore we have 6.938 x 10⁻⁴ moles of NaI and 6.938 x 10⁻⁴ moles Na⁺.
Step 5
Calculate molarity of sodium cation
Molarity = moles Na⁺ / volume