Answer:
dH = +90kJ
Explanations:
Given the following chemical reactions with their individual enthalpy change expressed as:
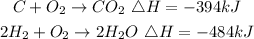
We need the enthalpy change value for the reaction shown:
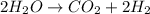
To get the equivalent reaction from the 2chemical reactions above, we will interchange the product and reactant of the formation of water to have:
![\begin{gathered} C+\cancel{O_2}\operatorname{\rightarrow}CO_2\operatorname{\triangle}H=-394kJ \\ 2H_2O\rightarrow2H_2+\cancel{O_2}\triangle H=+484kJ \\ _(--------------------------------------) \\ C+2H_2O\rightarrow CO_2+2H_2\text{ }\triangle H=-396kJ+484kJ=+90kJ \end{gathered}]()
Hence the dH value for the following chemical reaction C + 2H2O -> CO2 + 2H2 is +90kJ