We are given the following information:
110 oz of 60% acid solution
We need to find the amount of water needed to dilute it to 45%.
To answer this, let's set up the equation first.
We know that 60% of the 110 oz. is acid. This amount of acid must then be the same amount of acid that makes up 45% of the new solution. So our equation would be:
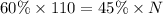
Solving for N, we get:
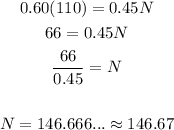
Now we know that the total amount must be 146.67 oz. We already have 110 oz. So we only need 146.67 - 110 = 36.67 oz of water to dilute the solution.