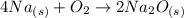
Firstly we convert the mass of Na to moles of Na:
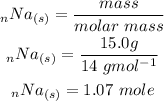
Based on the mole ratio 4 moles of Na reacts with 1 mole of O2. Therefore 1.07 mole of Na would react with x mole of oxygen gas. We determine x by:
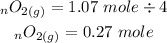
We will now convert 0.27mol of oxygen gas to mass of oxygen gas:
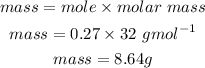
Mass of O2 is 8.64g