ANSWER and EXPLANATION
We want to identify the parameters of the line in the electromagnetic spectrum.
The wavelength of line c is given as 486.13 nanometers. Converting this to meters, we have that the wavelength of line c is:
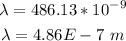
To find the frequency of line c, apply the relationship between the velocity of light and wavelength:
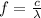
where c = velocity of light in a vacuum
f = frequency of the light
Hence, the frequency of line c is:
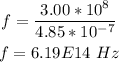
The velocity of light in a vacuum is given by:
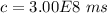
The energy of the line can be found using the formula:
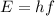
where E = energy
h = Planck's constant = 6.63E-34 Js
Hence, the energy of line c is:
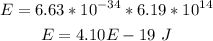
Those are the answers.