Answer:
180 g/mol.
Step-by-step explanation:
What is given?
Molar mass of C = 12 g/mol.
Molar mass of O = 16 g/mol.
Molar mass of H = 1 g/mol.
Step-by-step solution:
To calculate the molar mass of a compound, we have to know the molar masses of the elements that conform to this compound. The molar masses can be found in the periodic table and, in this case, our elements that conform to C6H12O6 are C (carbon), H (hydrogen), and O (oxygen).
Then, we have to do an algebraic sum based on the quantity of each element in the compound. You can realize that in C6H12O6 we have 6 carbons, 12 hydrogens, and 6 oxygens, so we have to multiply each of these numbers by their respective molar mass and sum everything. This will look like this:
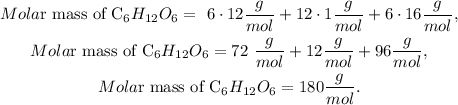
So the molar mass of C6H12O6 is 180 g/mol.