The mass percentage of each element in a compound is defined by:
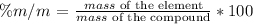
The addition of all the mass percentages has to be equal to 100%.
Then, if we only have 2 elements and we know the mass percentage of one of them, we can find the other one through a subtraction:
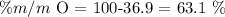
Then, the oxygen mass percentage is equal to 63.1%