You can see that in the balanced equation 2 moles of sodium thiosulfate produce 1 mole of NaBr. So first, let's find the number of moles of 64.3 g of NaBr using its molar mass which is 102.9 g/mol:
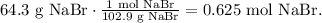
With this value, we can find the number of moles of Na2S2O3 using a rule of three:
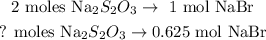
And we're going to obtain:
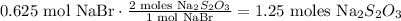
And the final step is to do the conversion from moles to grams using the molar mass of sodium thiosulfate which is 158 g/mol:
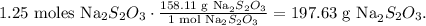
The answer is that we need 197.6 grams of sodium thiosulfate to produce 64.3 grams of NaBr.