INFORMATION:
We know that:
- A solution of ammonia has a pH of 11.8
And we must find the concentration of OH– ions in the solution
STEP BY STEP EXPLANATION:
To find it, we need to know that pH & pOH are related by the following equation
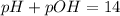
Now, using the given information, we know that pH = 11.8
Then, replacing the value in the formula
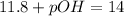
Solving for pOH,
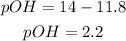
The concentration of OH- ions is given by
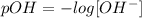
Replacing the value of pOH,
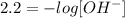
Finally, solving for OH-
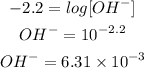
Hence, concentration of OH- is 6.31 x 10^-3 M
ANSWER:
6.31 x 10^-3 M