Answer:
18 electrons
Explanations
Shells of an element are denoted using letters K, L, M N, O, P... The expression for calculating the number of electrons in the shell of an element is expressed as:
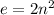
where:
n is the number of shells
Given the following parameters:
n = 3
Substitute the given value of "n" into the formula to have:
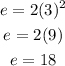
Hence the maximum number of electrons that may be placed in the third shell is 18 electrons