INFORMATION:
We have the following options:
- 6
- 18
- 12
- 24
And we must select the one which is the mass number of Carbon–12 with 6 protons and 6 neutrons
STEP BY STEP EXPLANATION:
To select the correct one, we must know that:
So, we can calculate the mass number as
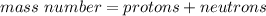
In our case, there are 6 protons and 6 neutrons.
So,
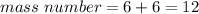
Finally, the mass number of Carbon–12, which has 6 protons and 6 neutrons is 12
ANSWER:
12