In this case we are given the percentage of each element in a sample, and we know that the molecular mass is 250 amu.
The amu unit is defined as:
1/12 of the mass of an unbound neutral atom of carbon-12.
And 1 mol of uma equals 1g.
So in this case knowing that 1 molecules has a weight of 250 uma, we can calculate the molar mass (g/mol), which is 250g/mol.
Now, we start in step 1.
Assuming that we have 100g of the substance, using the percentage given we calculate the mass (mx) of each element using the following formula:
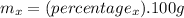
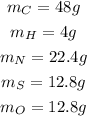
Step 2
To convert the number of grams of each element into moles we use the molar mass (M) of each of them, this information we obtain from the periodic table:
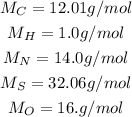
So we calculate the moles (n) as follows:
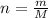
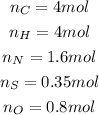
Step 3
We divide the number of moles of each element by the smallest number obtained:
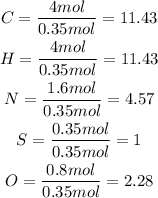
Steps 4 and 5
To calculate the empirical formula, we use