Answer:
0.688 moles of H.
Step-by-step explanation:
What is given?
moles of oxygen (O) = 0.344 moles.
molar mass of oxygen (O) = 16 g/mol.
molar mass of hydrogen (H) = 1 g/mol.
Chemical equation:
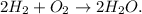
Step-by-step solution:
In the statement, they're telling us that we have 0.344 moles of O, but since we have a compound of two oxygens as the reactant, then we have 0.688 (0.344 x 2 =0.688) moles of O2. So let's see how many moles of H2 we need to react with 0.688 moles of O2. You can see that 2 moles of H2 react with 1 mol of O2:
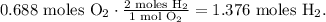
But the problem requires the number of moles of H, not H2, so we can divide the number of moles of H2 obtained by two:
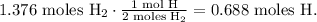
The answer is that we need 0.688 moles of H to react with 0.344 moles of O.